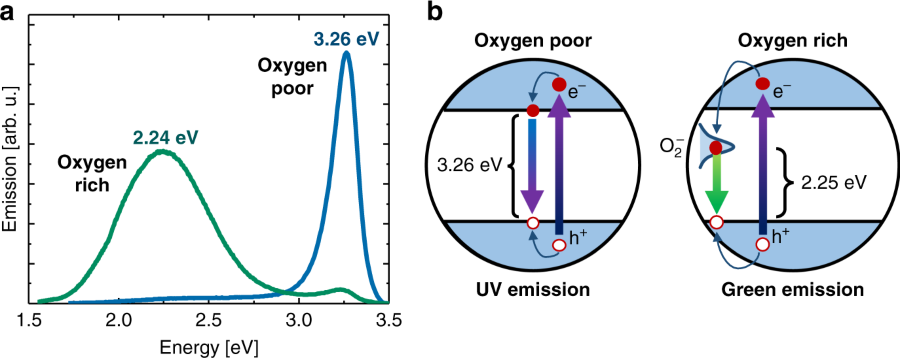
Zinc oxide is a versatile semiconductor with an expansive range of applications including lighting, sensing and solar energy conversion. Two central phenomena coupled to its performance that remain heavily investigated are the origin of its sub-band-gap green emission and the nature of its conductivity. We report photoluminescence and dark conductivity measurements of zinc oxide nanoparticle films under various atmospheric conditions that demonstrate the vital role of adsorbates. We show that the UV emission and conductivity can be tuned reversibly by facilitating the adsorption of species that either donate or extract electrons from the conduction band. When the conductivity data are compared with photoluminescence spectra taken under the same ambient conditions, the green emission can be directly linked to surface superoxide formation, rather than surface hydroxylation or native defects such as oxygen vacancies. This demonstrates how and explains why the green emission can be controlled by surface reactivity and chemical environment.